

It is shown that brief exposure to a low-frequency magnetic field at the stage of release of the esterases in the course of swelling of wheat seeds greatly speeds up the release of enzymes from the bound state and also the release of seeds from the dormant state. Later, the field effects appreciably dropped. In some experiments the magnetic field produced an earlier pH shift close to the germ. In old seeds the activity maximum of the esterases was observed later. Their exposure to a magnetic field did not change or lower the release into the medium of the products of esterase activity which is ascribed to the influence of the processes induced by it on the recovery of the barrier function of the membranes. Consistent with this is the absence in the experiments of microflora growing in the control on the products of leakage from old seeds. The germinating power and the rate of germination of such seeds also sharply increased.
© 1997 Elsevier Science Ltd. All rights reserved.
Despite the presence of extensive literature on the effects of non-thermal exposure of biological systems to electromagnetic fields and the growing interest in such research, the problem itself continues to remain debatable connected not only with the difficulties of interpreting the results in conditions when the energy of the possible transitions under the influence of an electromagnetic field (e.m.f.), especially at low frequencies, is many orders lower than kT, but also with the complexity of isolating the effects of an e.m.f. in the experiment. They depend on the state of the organism, which cannot always be controlled; in the organism a host of different processes occurs in parallel and these may react in different directions to an e.m.f., possibly with other unheeded weak influences etc., reducing the reproducibility of the data and their reliability. Therefore, an important role is played by the choice of the objects for the investigations and the conditions for running them, which would limit the influence of such factors. The design of the experiment and the measured indicators must also be correlated with the possibility of verifying the particular models of the influence of an e.m.f. on biological processes.
Taking all this into account, for the study of e.m.f. effects, we chose wheat seeds at the initial stages of germination. The choice of plant seeds was dictated by the fact that their passage from the resting state to germination is linked with a definite sequence of processes [1] and by selecting the time of exposure of these processes to the e.m.f., one may, in principle, selectively influence particular reactions. In turn, the different sensitivity to an e.m.f. in different processes in the course of seed germination permits differential measurements, raising the reliability of recording the e.m.f. effects. Also of great importance for elucidating its mechanisms is the use in the experiments of seed batches differing in germinating power and the kinetics of germination and also in the possible biological effects.
In looking at the probable mechanisms of the impact of an e.m.f. on biological systems, we started from the premise that one of the processes most sensitive to external agents is the transitions of different proteins, in particular peripheral, from the membrane-bound state to the aqueous medium. Such unidirected processes occur at certain stages of release of the seeds from the resting state. Such transitions of proteins, because of the rise in the number of degrees of freedom for the protein groups in an aqueous medium and, accordingly, the entropy of the system, must be related to minor change in free energy [2]. They may be caused by local changes in pH or ionic strength or the concentration of Ca2+ ions sensitive to the influence of the e.m.f., the modelling data showing that the e.m.f. effects in the low-frequency region (from O.I to 102 Hz) may be significantly enhanced through non-linear processes in the near-membrane layer [3, 4].
As parameters sensitive to the action of a low-frequency e.m.f., besides the biological indices (germinating power, rate of germination of wheat seeds, etc.), we chose change in pH in the direct vicinity of the seed germ and change in the hydrolytic activity of the esterase enzymes released at the early stages of germination of wheat seeds.
The main objects for the investigation were Zarya wheat seeds of the 1991 harvest (germinating power ~95%) and, for comparison, the same of the 1987 harvest, the germinating power of which, without treatment, was about 20%. We also used other wheat seed varieties with different germinating power. The seeds were swollen in Petri dishes by the standard technique. Each experiment was run as test and control variants with simultaneous wetting of the seeds. Then the test sample in an interval between I and 30 h after the start of wetting was subjected once in 7-10 min to the action of a low-frequency rotating magnetic field, the source of which was the MM-5 magnetic mixer. The dish with seeds was usually placed on the surface of the mixer, but in individual experiments at different distances from it. The maximum amplitude of the varying magnetic field at the site of the sample on the surface of the mixer was about 30 mT at a frequency of 30-33 Hz and for a form close to sinusoidal.
As biological indicators we used change in the germinating power of the seeds and the total weight of the seedlings (on conversion to 10 seeds) after exposure to the magnetic field indicated, usually 6 days from the start of swelling. The changes in the development of the microflora on the seeds in the course of their germination also served as an indirect pointer.
The activity of the esterase enzymes in the course of swelling of the seeds of wheat and change in it after treating the seeds with the magnetic field were determined from the efficiency of hydrolysis to fluorescein of the non-fluorescing substance fluorescein diacetate (PDA). Ten wheat seeds each of the control and test variants at the chosen stage of swelling were washed three times in water to remove the earlier released leakage products and wetted with 3 ml water, to which was added 15 l 0.5% aqueous solution of PDA. After 50 min, samples were taken, each of 200 1 solution. The microfluorimetric measurements were made with the LYUMAM-IZ luminescent microscope with the FMEL I A photometering cap for a diameter of the photometered region 150 rn. To excite fluorescence, we used the KGM 9-70 lamp and the SZS 21-2 and FS 1-2 light filters and to register it, an interference light filter with a transmission maximum at 520 nm and half-width 12 nm, the FEU-79 photomultiplier and a digital voltmeter.
Measurements of pH at the germ surface in the course of swelling of an individual wheat seed were made with an antimony end microelectrode in glass insulation with a tip diameter 10-20 m, the position of which, was changed through a step mechanism with a step of 1 m and controlled with a microscope [5]. The microelectrode tip was situated 5-10 m from the germ surface, which allowed us to determine the local pH changes at the earlier stages of swelling of the seeds before their development in volume.
Measurements of the activity of the esterases in the course of
swelling of wheat seeds with high-germinating power showed that
in the first hours the rate of hydrolysis of PDA was roughly constant
but, starting from hours 7-8, it appreciably increased. The activity
maximum was reached between hours 15 and 18, but later dropped
to the original value (Fig. I ). In the seeds with low-germinating
power after an initial high rate of hydrolysis, it fell, probably
related to release of the enzymes and products of hydrolysis of
FDA from the damaged cells. Then the rate of hydrolysis rose and,
as in the first seeds but 10-12 h later, reached its maximum (Fig.
1).
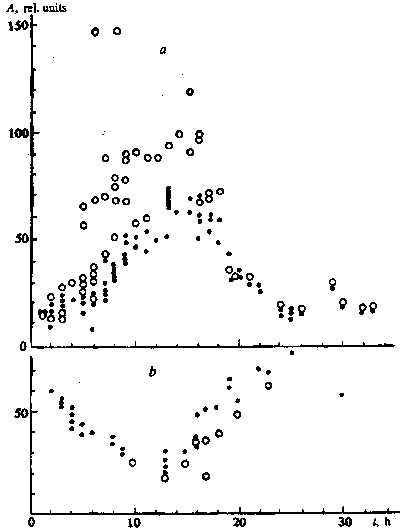
Fig. 1. Rate of decomposition of FDA by the esterases as a function
of the time of swelling (t) of wheat seeds of the Zarya variety
with high (a) and low (b) germinating power without field exposure
() and after 7-min exposure to a low-frequency magnetic field
(o)
Brief treatment of the wheat seeds by the magnetic field in the first hours of swelling had relatively little influence on the rate of hydrolysis of FDA. But then at the stage of growth in the activity of the esterases in the seeds with high-germinating power, the field effects sharply increased, by a factor of two and more in individual experiments. In the interval from hours 6 to 11, two groups of experiments are singled out - with a strong and, in a smaller number, with a weak reaction to the magnetic field After 11 h, strong effects were noted in all tests and, starting from hours 17-18, the magnetic field weakly influenced the rate of hydrolysis. At the same time in old seeds the exposure to magnetic field either did not change or lowered release into the medium of the products of hydrolysis of FDA (Fig. 1).
The measured effects weakly depended on the site of the sample on the surface of the mixer. When it was inclined by I mm in relation to the ends of the rotating magnet 50 mm long and the sample with a diameter of 40 mm was placed at the centre and at the ends of the magnet, the mean rates of hydrolysis for 16 tests were close to each other and in relation to the centre equal to 1.12 ± 0.08 in the larger and 1.10 ± 0.06 in the smaller field.
Additional information on the mechanisms of action of the magnetic
field is provided by measurements of pH close to the germ surface.
In the untreated seeds considerable changes in pH, associated
with evacuation of protons from the medium for acidifying the
cells [1], were observed approximately one day after the start
of swelling (Fig. 2), as was recorded in all 10 tests and, likewise,
after exposure of dry seeds to the magnetic field (Fig. 3). But
in the swelling seeds in some tests with a delay of several hours,
treatment by magnetic field accelerated the process by a factor
of two and more (Figs 2 and 3). The reaction of the different
seeds to the magnetic field varied. And, although after exposure
to the magnetic field between hours 3 and 11 clearly marked effects
were present only in 4 of 10 tests, they could not be rated random;
rather they reflected heterogeneity of the seed population. This
follows from measurements of the pH gradient between the near-surface
layer of the germ and at a distance of I mm from it made for the
magnetic field-treated seeds for 6-h swelling and for the control
seeds 21 h after wetting. The different pH values for 10 seeds
of this and another group was 0.123 units, with in both groups
seeds giving a slight pH gradient. But for the five most active
seeds in each of the groups, the pH value for the treated seeds
exceeded that for the control by 0.23 ± 0.07 units.
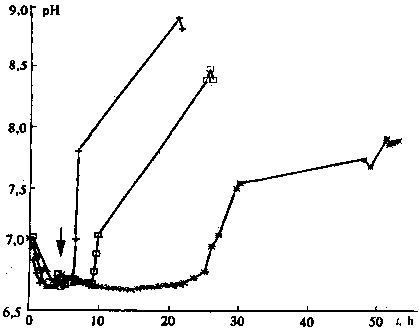
Fig. 2. Kinetics of change in pH close to the germ of the wheat
seed without field exposure (*) and after 10 min. exposure to
low-frequency magnetic field () and (+). The exposure time is
shown by the arrow.
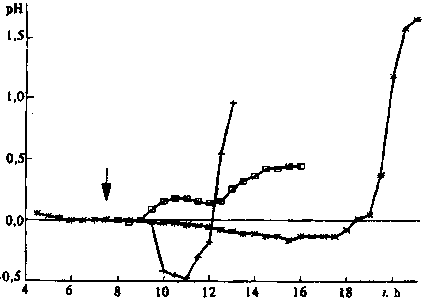
Fig. 3. Same for the relative change in pH after exposure to magnetic
field of dry seeds (*) and swelling wheat seeds () and (+).
The results indicate activation of metabolism on exposure to a
low-frequency magnetic field in a series of stages of swelling
of wheat seeds, as confirmed by recording notable changes in their
germinating power and rate of germination, particularly distinctly
marked in seeds with low germinating power. The degree of the
effect depended on the chosen swelling time and was highest in
the interval between hours 12 and 22 after its start. From the
results, to study the effects of the magnetic field, we chose
a 17-h interval. The results of the experiments conducted for
2 years in different seasons are presented in Table I, from which
it follows that in nearly all cases treatment with a varying magnetic
field raises the germinating power and rate of germination of
the seeds. Another significant effect is the absence in magnetic
field-treated seeds of mould, usually developing in the leakage
products from old seeds after swelling for several days. Its absence
in the experiments was also noted for seeds with zero germinating
power, excluding the seeds of the Albidum variety of the 1971
harvest. At the same time, in the old seeds in the control, we
observed active development of mould (Table I does not give all
the cases, since it gives pride of place to the test seeds).
| Seed variety | % Germination | Weight of seeidlings, mg | Presence of mould | Control | Test | Control | Test | Control | Test |
| Zarya | 100 | 100 | 671 | 801 | - | - |
| Zarya | 90 | 100 | 478 | 625 | - | - |
| Zarya (4 days) | 100 | 100 | 189 | 437 | - | - |
| Zarya (old) | 10 | 50 | 22 | 271 | + | - |
| Zarya (old) | 10 | 40 | 20 | 244 | + | - |
| Zarya (old) | 20 | 60 | 105 | 431 | - | |
| Zarya (old) | 40 | 100 | 180 | 766 | - | |
| Zarya (old)* | 0 | 0 | 0 | 0 | + | - |
| Zarya | 87 | 90 | 465 | 505 | - | |
| Zarya** (5 days) | 10 | 10,25,20 | 94 | 54,259,182 | + | -,-,- |
| Inna | 70 | 90 | 188 | 455 | - | |
| Inna | 80 | 90 | 406 | 498 | - | |
| Steppe 7 | 80 | 90 | 396 | 497 | - | |
| Tarasovskaya (4 days) | 60 | 70.90,90 | 421 | 422,539.543 | + | -.-.- |
| 1 iral'skaya | 0 | 0 | 0 | 0 | + | - |
| Al'bidum | 0 | 0 | 0 | 0 | + | + |
| Donskaya (4 days) | 0 | 0,0.0 | 0 | 0,0,0 | + | -,-,- |
| Inna (7 days) | 70 | 90,90,80 | 647 | 680,738,664 | + | -,-,- |
| Note: *After 2 years; **Seeds artificially aged at 40°C for 7 days. | ||||||
To exclude the possible effects of vibration and to study the dependence of the biological effects on the size of the magnetic field, we ran experiments with the seeds in different positions in relation to the surface of the mixer. In six experiments carried out with different seeds, the weight of 10 seedlings in the control was 331 ± 59 mg, for the seeds on the surface 548 ±47, 15 mm away from it 468 ± 48, and 35 mm away from it 499 ± 40 mg, i.e. in all cases the effects went beyond the scatter of the measurements and, as in the other test samples, mould was absent.
Thus, the findings point to the high sensitivity of wheat seeds in the course of their germination to exposure to a low-frequency magnetic field Such sensitivity appears at the stage when the conditions are created for the passage of a number of proteins from the bound state to water and here magnetic field exposure additionally stimulates this process. A factor directly influencing such transitions appears to be a change in pH observed in the tests on exposure to a low-frequency magnetic field, which, in general, confirms the data of the mathematical model [3, 4]. The lag noted in the experiments with change in pH in the medium close to the germ may be connected with both the use at the first stages of a more acidic medium in the cell wall as compared with the cell and the kinetics of development of the process after exposure to magnetic field
Change in pH and liberation of proteins hasten the release of the seeds from the resting state and probably stimulate development of the restorative processes in the seeds. It is known that membrane damage, for example, on insertion of the electrode, leads to rise in the viscosity of the cytoplasm, with which, in turn, is linked healing of its lesions [6, 7]. Such a process of rise in the viscosity of the cytoplasm also occurs in the transitions of proteins from the bound state and, on the basis of this, one can fully explain [2] the known data on rise in the viscosity of the cytoplasm and also other phenomena attending this process [6. 7]. This suggests that sharp stimulation of the transitions of the proteins from the bound state under the influence of magnetic field treatment, apparently also induces the processes of healing of damage to the membranes and recovery of their barrier function, leading to the fall observed in the experiments in the release of the products of hydrolysis of FDA from old seeds after magnetic field exposure. The recovery of the barrier function of the membranes also determines the rise in the germinating power of old seeds and the absence in them of a microflora, usually growing on the products leaking from the seeds. Also linked with activation of the process of recovery, together with activation of the enzyme systems, is rise in the rate of germination of such seeds.
1. J. D. Bewley and M. Black, Physiology and Biochemistry of Seeds in Relation to Germination, Vol. 1. Development, Germination and Growth. Springer, Berlin (1978).
2. S. 1. Aksenov, Water and its Role in the Regulation of Biological Processes, Nauka, Moscow (1990).
3. G. Yu. Riznichenko, T. Yu. Plyusnina, T. N. Vorob'eva, S. 1. Aksenov and G. M. Chemyakov, Biofizika, 38, 667 (1993).
4. G. Yu. Riznichenko, T. Yu. Plusnina and S. 1. Aksyonov, Bioelectrochem. Bioenerg., 35, 39 (1994).
5. D. Remis, A. A. Bulychev and G. A. Kurella, Biochim. Biophys. Acta, 852, 633 (1986).
6. L. Heilbrunn, Dynamics of Living Protoplasm, Inostr. lit., Moscow (1957).
7. V. Ya. Aleksandrov, The Reactivity of Cells and Proteins,
Nauka, Leningrad (1985).