МОДЕЛЬ МИТОХОНДРИАЛЬНОЙ КАЛЬЦИЙ-ЗАВИСИМОЙ
ПОРЫ: ВОЗМОЖНАЯ РОЛЬ КОМПЛЕКСОВ КАЛЬЦИЯ С ПАЛЬМИТИНОВОЙ КИСЛОТОЙ
Миронова Г.Д., Gateau-Roesch O., Levrat C.,
Гриценко Е., Павлов Е., Лазарева А.В., Лимаренко Е., Rey C., Louisot P., Saris
N.-E., Agaphonov A.
(Пущино, Lyon, Helsinki)
Показано, что пальмитиновая и стеариновая кислоты имеют гораздо более высокое сродство к Ca2+ по сравнению с другими липидами. Обнаружено, что в гидрофобном окружении эти кислоты связываются с Са2+ координационными связями в соотношении 8:1. Добавление Са2+ к БЛМ, содержащей пальмитиновую или стеариновую кислоту, ведет к появлению неспецифичной проводимости проводимости канального типа. Получены доказательства, что комплексы Са2+ с длинноцепочечными насыщенными жирными кислотами участвуют в образовании митохондриальной Ca2+-зависимой поры, что объясняет проапоптотический эффект пальмитиновой кислоты.
A MODEL OF
MITOCHONDRIAL PERMEABILITY TRANSITION PORE: A POSSIBLE ROLE OF PALMITIC
ACID/CALCIUM COMPLEXES
Mironova G.D.1, Gateau-Roesch O.2, Levrat C.2, Gritsenko E.1, Pavlov E.1, Lazareva A.V.3, Limarenko E.1, Rey C.2, Louisot P.2, and Saris N.-E.4
(Pushchino, Lyon, Helsinki)
It has been shown that palmitic and stearic acids have their affinity to Ca2+ being much higher than that of many other lipids. In hydrophobic environment, these acids have been found to attach to Ca2+ by coordinate bonds with a ratio of 8:1. Addition of Ca2+ to BLM, containing palmitic or stearic acid, lead to the appearance of a non-specific channel-like conductivity. Evidence has been obtained that complexes of Ca2+ with long-chain saturated fatty acids are involved into formation of the mitochondrial permeability transition pore, this explaining the pro-apoptotic effect of palmitic acid.
Introduction
Palmitic acid (PA) has recently been shown to promote apoptosis through the release of pro-apoptotic factors from mitochondria (De Pablo et al., 1999; Kong and Rabkin, 2000). A little is known on the mechanism of PA action, excepting a general notion that PA induces the dissipation of mitochondrial transmembrane potential (DY). An open question is either PA acts somehow indirectly or it is involved into formation of the mitochondrial permeability transition (MPT) pore, responsible for the release of pro-apoptotic factors. Although these are protein complexes that are generally accepted to assemble the pore (Zoratti and Szabó, 1995), a role of lipids also might be important. The key point in studying MPT is to determine “receptors” for Ca2+, as in various ways of MPT induction, Ca2+ remains an invariant reqired factor. In this paper we demonstrate that PA and stearic acid (SA) bind Ca2+ with an affinity, much higher than that of other fatty acids and lipids. The paper considers the nature and properties of Ca2+-PA/SA complexes and their possible role in MPT pore formation.
Materials and Methods
Most of the procedures used in the present work were described earlier, so we confine only to their list with references. For experimental conditions see figure legends.
|
Isolation of rat liver mitochondria |
Gateau-Roesch et al., 2000 |
|
Isolation of site-enriched fractions from mitochondria |
Ardail et al., 1990 |
|
Assay of cytochrome c oxidase activity |
Wharton and Tzagoloff, 1967 |
|
Assay of monoamine oxidase activity |
Caman et al., 1965 |
|
Extraction of lipids from mitochondrial membranes |
Gateau-Roesch et al., 2000 |
|
Detection of fatty acids using gas chromatography |
Ardail et al., 1990 |
|
Binding of 45Ca2+ with lipids and fatty acids |
Gateau-Roesch et al., 2000 |
|
Estimation of parameters for binding of Ca2+ with lipids and fatty acids |
Motulsky, 1995 |
|
Transport of ions through BLM |
Mueller et al., 1962 |
|
Measurement of mitochondrial DY using a TPP+-sensitive electrode |
Kamo et al., 1979 |
Results
Ca2+-binding
properties of fatty acids and lipids
The data of Table I indicate that saturated fatty acids (SFA) have higher Ca2+-binding capacity than unsaturated ones, phospholipids, and lysophospholipids. The affinity of SFA to Ca2+ varies with the length of the aliphatic chain, with PA, SA and eicosanoic acid exhibiting the highest Ca2+-binding capacity.
Table 1. Ca2+-binding properties of fatty acids and lipids*
|
Compound |
Relative Ca2+ binding, % |
|
Lauric acid (12:0) |
0.50 ± 0.03 |
|
Myristic acid (14:0) |
7.30 ± 0.25 |
|
Palmitic acid (16:0) |
83.00 ± 0.72 |
|
Stearic acid (18:0) |
100.00 ± 0.00 |
|
Eicosanoic acid (20:0) |
73.00 ± 2.50 |
|
Docosanoic acid (22:0) |
44.00 ± 1.20 |
|
Lignoceric acid (24:0) |
15.00 ± 0.35 |
|
Palmitoleic acid (16:1) |
1.90 ± 0.08 |
|
Oleic acid (18:1) |
5.70 ± 0.12 |
|
Linoleic acid (18:2) |
0.65 ± 0.04 |
|
Linoleinic acid (18:3) |
0.87 ± 0.05 |
|
Arachidonic acid (20:4) |
1.10 ± 0.05 |
|
1-Palmitoyl-lysophosphatidylcholine |
0.43 ± 0.02 |
|
1-Stearoyl-lysophosphatidylcholine |
0.47 ± 0.02 |
|
1-Lauroyl-lysophosphatidylcholine |
0.43 ± 0.01 |
|
Lysophosphatidylserine |
0.54 ± 0.03 |
|
1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine |
0.40 ± 0.20 |
|
1,2-Dipalmitoyl-sn-glycero-3-phosphatidylethanolamine |
0.22 ± 0.01 |
|
1-Palmitoyl-sn-glycero-1-3-phosphatidylethanolamine |
0.76 ± 0.03 |
|
Palmitoyl-CoA |
0.43 ± 0.02 |
|
Cardiolipin |
0.60 ± 0.03 |
|
L-α-phosphatidic acid |
19.50 ± 0.80 |
|
Cholesterol |
0.33 ± 0.01 |
|
Cerebrosides |
0.20 ± 0.01 |
|
Sphingomyelin |
0.30 ± 0.01 |
* 5 µl of 2 mM solution of each compound were applied on a PVDF membrane, and the binding of Ca2+ to the sample was estimated at pH 8.5 in the presence of 5 µM 45CaCl2. Stearic acid was taken as a reference to calculate the relative Ca2+ binding.
Ca2+-Binding
Properties of Palmitic and Stearic Acids
|
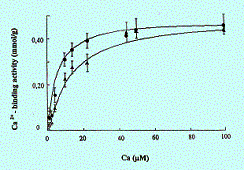
Fig. 1. Binding of Ca2+ with PA at pH 8.5 (upper curve) and pH 7.5 (lower curve) |
Binding of Ca2+ with PA and SA is found to be pH-dependent with a maximum at pH 8.5 (data not shown). In Fig. 1, presented are concentration curves of Ca2+ binding with PA measured at pH 7.5 and 8.5. Calculation of Kd and Bmax for these curves yields the following values: 5 mM at pH 8.5 and 15 mM at pH 7.5 for Kd; 0.48 ± 0.08 mmol of Ca2+ per g of PA at both pH for Bmax. Considering the molecular weight of PA to be 256 Da, one can obtain the maximal PA/Ca2+ ratio of 8:1. Therefore, up to eight molecules of PA would bind to a Ca2+ ion.
Apparently, the bonds between PA molecules and a Ca2+ ion are coordinate. It is known that in coordinate complexes Ca2+ will have 6–8 bonds (Williams, 1976). Evidence of Ca2+-PA complexes being of coordinate nature comes from the results of IR-spectroscopy (data not shown). The IR-spectrum of PA, measured under anhydrous conditions, changes in the presence of Ca2+. The alterations observed indicate the formation of PA-Ca2+ complexes (appearance of a more narrow and intensive band at 3403 cm-1), but there is no signs of salt bonds between PA and Ca2+ (the band of carbonyl group does not shift from 1705 to 1558 cm-1).
Binding of Ca2+ with PA is inhibited by physiological concentrations of Mg2+ (0.1-1 mM), but not affected by 50–500 mM K+.
Results, similar to that demonstrated for PA, have been obtained also for SA (data not shown).
Influence of palmitic
and stearic acids on BLM permeability
As it follows from the data of Table 2, incorporation of PA or SA into BLM did not change the level of membrane conductance. But the addition of Ca2+ to BLM, formed from total brain phospholipids/cardiolipin mixture and containing PA or SA, resulted in a 30-fold increase in membrane permeability. It was not merely a rupture of the membrane, for it was able to work for an hour or more. The subsequent addition of 100 mM KCl caused the further growth of conductance. When mitochondrial lipids were used for BLM formation, the same effects could be achieved at a smaller content of PA or SA in the membrane and at a lower concentration of Ca2+ in the medium. In the presence of the 3-fold Ca2+ gradient, the membrane potential was only 3 mV, this indicating the permeability to be non-specific. In contrast to PA and SA, palmitoleic acid, which had an unsaturated bond and bound Ca2+ with low affinity, did not alter the membrane permeability in the presence of Ca2+ and K+.
Table 2. Influence of fatty acids on BLM permeability*
|
|
Additions |
BLM conductance, ´ 10-9 Sm/cm2 |
||
|
Palmitic acid |
Stearic acid |
Palmitoleic acid |
||
|
Total brain lipids |
Control |
1.0-1.5 |
1.0-2.0 |
1.0-1.6 |
|
0.1 mM CaCl2 |
1.0-1.5 |
- |
- |
|
|
0.5 mM CaCl2 |
10.0-50.0 |
28.5-53.1 |
- |
|
|
1 mM CaCl2 |
30.0-150.0 |
53.1-114.6 |
1.1-3.0 |
|
|
0.1 mM CaCl2 + 30 mM KCl |
10.0-12.0 |
- |
- |
|
|
1 mM CaCl2 + 30 mM KCl |
- |
122.1-341.6 |
1.1-1.9 |
|
|
1 mM CaCl2 + 100 mM KCl |
300.0-500.0 |
- |
- |
|
|
100 mM KCl |
1.0-1.5 |
1.0-2.0 |
- |
|
|
Mitochondrial lipids |
Control |
4.1-6.1 |
2.0-6.1 |
- |
|
0.1 mM CaCl2 |
10.2-14.3 |
20.4-30.6 |
- |
|
|
0.5 mM CaCl2 |
40.8-48.9 |
- |
- |
|
|
1 mM CaCl2 |
581.0-618.0 |
- |
- |
|
|
0.1 mM CaCl2 + 30 mM KCl |
51.0-81.0 |
57.1-114.6 |
- |
|
|
1 mM CaCl2 + 30 mM KCl |
15600-30000 |
- |
- |
|
|
1 mM CaCl2 + 100 mM KCl |
40700-40900 |
- |
- |
|
|
100 mM KCl |
6.5-9.0 |
6.1-10.2 |
- |
|
* BLM was formed from the following mixture: 85.5% of total brain or mitochondrial lipids, 10% of cardiolipin and 0.5% of fatty acid (w/w), dissolved in n-decane.
Localization of Ca2+-binding
domains in the lipid matrix of the inner mitochondrial membrane
After mitoplasts being subjected to the hypotonic treatment, the centrifugation of the suspension in a discontinous sucrose gradient yielded two fractions. The first fraction (IM1) was enriched in monoamine oxidase and contained a high level of cholesterol, so it may be considered as a contact site-enriched fraction (Levrat and Louisot, 1992). The second fraction (IM2) was enriched in cytochrome c oxidase and was adopted to be an inner membrane-enriched fraction. As appeared, the purified SFA-containing extract from IM1 had a much higher Ca2+-binding capacity comparing with the capacity of that from IM2 (Table 3). Thus, free SFA, which determine the Ca2+-binding capacity of the lipid extract, would be mainly located in the contact sites of the inner mitochondrial membrane.
Table 3. SFA-dependent Ca2+-binding capacity of different fractions of the inner mitochondrial membrane
|
|
Contact site-enriched fraction |
Inner membrane-enriched fraction |
|
Monoamine oxidase activity, % of total |
81.5 |
18.5 |
|
Cytochrome oxidase activity, % of total |
21.6 |
78.4 |
|
Cholesterol content, mg/mg of protein |
6.54 |
2.85 |
|
Ca2+-binding capacity, % of total |
86 |
14 |
Relation of saturated
fatty acids to the mitochondrial permeability transition
It was shown that the addition of Ca2+ to mitochondria leads to the activation of phospholipase A2, this increasing the content of free fatty acids in the membrane (Pfeiffer et al., 1979). Our results were in good agreement with the data of Pfeiffer (data not shown). We also observed an increase in the content of free fatty acids in mitochondria after Ca2+ treatment. Accordingly, rising was the Ca2+-binding capacity of the partially purified SFA-containing fraction from mitochondrial lipid extract. The addition of aristolochic acid, an inhibitor of phospholipase A2, removed these effects. The Ca2+-induced increase of SFA content in the membrane occurred just at the moment when the MPT pore was about to open. This suggests a direct involvement of SFA in MPT, also confirmed by the fact that cyclosporin A (CsA), a specific inhibitor of pore, prevents Ca2+-induced accumulation of SFA in the membrane (Gateau-Roesch et al., 2000).
Discussion
The main finding of this work is that SFA, and first of all PA and SA, have a high affinity to Ca2+. When being in hydrophobic environment (particularly, in the membrane), SFA form complexes with Ca2+, in which up to 8 molecules of SFA attach to a Ca2+ cation by coordinate bonds. We guess, such complexes would have a form of inverted micelles submerged in the lipid bilayer. Indeed, the ability of Ca2+ to promote the appearance of non-bilayer lipid phases, including micellar one, in membranes is well-known (Borovyagin and Sabelnikov, 1989). It also cannot be excluded that under hydrophilic conditions, SFA molecules form dimers, inserted in the lipid bilayer, and Ca2+ ions would bind to these dimers at the membrane/water interface (Waters et al., 1984).
As it follows from our data, the affinity of SFA to Ca2+ varies with the length of their aliphatic chains. This may relate to the capability of SFA-Ca2+ complexes to be compactly embedded in the bilayer.
Obviously, formation of Ca2+-PA/SA complexes would cause perturbations in the membrane organization. The results obtained on BLM confirm this statement. One can observe ion leaks and formation of channels. This is not a rupture of the membrane, for it can work for an hour and more. It is worth to note that the conductance registered in the presence of PA/SA and Ca2+ cannot be ascribed to the ability of fatty acids to shuttle cations across the membrane. The channel-like conductance testifies rather to a disordering of the bilayer. The bilayer disordering is especially pronounced when mitochondrial lipids are used for BLM formation. In this case, a significant conductivity can be seen even at a small content of SFA in the membrane and at a low concentration of Ca2+ in the medium. Apparently, the composition of mitochondrial lipids is more favorable for the Ca2+-SFA complexes to form. It may also be supposed that some mitochondrial lipids incorporate with SFA in micellar phase.
Loading of mitochondria with Ca2+ should create all the necessary prerequisites for the Ca2+-SFA complexes to form in the inner mitochondrial membrane. First, with the concentration of Ca2+ inside mitochondria increased, the matrix pH will be alkalified (Saris, 1963), shifting to the optimum for binding of Ca2+ with PA (8.5). Second, both these factors, rising of Ca2+ concentration and alkalizaton of the matrix, will activate phospholipase A2. Phospholipase A2 begins to hydrolyze lipids and this would lead to the appearance of SFA-enriched domains or even “SFA lakes” in the bilayer. Ca2+-SFA complexes formed in mitochondria would predominantly be Ca2+-PA ones, as the content of PA is 2-3 times higher than that of SA, with eicosanoic acid presented only in a trace amount.
Our results indicate that formation of Ca2+-PA complexes in the contact sites of the inner mitochondrial membrane may relate to the opening of MTP pore. And there is quite a lot of literature data that are in agreement with this conclusion. Indeed, induction of MPT by non-esterified long-chain fatty acids was shown in several laboratories (Petronilli et al., 1993; Broekemeier and Pfeiffer, 1995; Schönfeld and Bohnensack, 1997). SFA were considered to act as weak protonophores, decreasing DY and thereby promoting MPT (Skulachev, 1991; Zoratti and Szabó, 1995; Bernardi, 1999). At the same time, Wieckowski and Wojtzsak found that classical uncoupling agents were ineffective in the presence of Ca2+, whereas myristic acid (a long-chain SFA) induced ADP- and CsA-sensitive swelling under these conditions (Wieckowski and Wojtzsak, 1998). Our data throw some light on the mechanism by which SFA can decrease DY in the presence of Ca2+. And such a decrease of DY may actually cause the dissociation of cytochrome c from the membrane and its subsequent release from mitochondria, this accounting for the pro-apoptotic effect of PA. However, is the decrease of DY the only effect that can explain SFA-induced MPT pore opening?
As generally accepted, MPT pore is a megachannel, consisting of several proteins. The conformational changes of one of this proteins, adenylate translocator (ANT), are supposed to produce a hypothetic hole in the megachennel, which is, in fact, regarded as the pore (Zoratti and Szabó, 1995; Bernardi, 1999). ANT is assumed to have Ca2+-binding sites, so the necessity in Ca2+ for the pore to open is explained by Ca2+-induced conformational changes of ANT. At the same time, ANT is known to have rather high specificity to SA (Horvath et al., 1990) and, possibly, PA (Schönfeld and Bohnensack, 1997). So SFA may be a part of the megachannel, playing a role of “Ca2+-sensors” for ANT.
However, remaining unclear is the question, why mitochondria are to have accumulated a certain amount of Ca2+ inside before the pore will open? We assume that this may be due to a necessity of SFA to accumulate in the membrane. There is a ceratin lag in the matrix alkalization and, accordingly, in the activation of phospholipase A2. The lag depends on the total Ca2+-binding capacity of mitochondria, as the concentration of free Ca2+ in the matrix (and, therefore, pH) would rise only after the Ca2+-binding capacity will have been exhausted. Thus, it seems that for the MPT pore to open, a certain amount of Ca2+-SFA complexes has to accumulate in the inner mitochondrial membrane. This is confirmed by the fact that the specific inhibitor of MPT, CsA, and the inhibitor of phospholipase A2 prevent the accumulation of Ca2+-SFA complexes (Gateau-Roesch et al., 2000). Therefore, in our mind, the mechanism of pore opening may be the following. Growth of Ca2+ concentration in the matrix and the corresponding increase of pH activate phospholipase A2, this leading to the rise of SFA amount and, accordingly, the amount of Ca2+-SFA complexes. As a result, an increase of non-specific permeability of the membrane is observed, which can be responsible for the opening of MPT pore.
It is known that the incubation of multilayer liposomes with Ca2+, accompanied by lipid polymorphic phase transitions, can lead to the appearance of numerous contacts between adjacent bilayers and their subsequent fusion (Borovyagin and Sabelnikov, 1989). A kind of this might take place in the mitochondrial contact sites. Indeed, all should favor to the arising of a gap junction between the inner and the outer mitochondrial membranes. On the one hand, complexes of Ca2+ with PA dimers inserted into the inner leaflet of the membrane should increase the lateral pressure along the membrane surface, promoting the formation of membrane folds. On the other hand, micellar Ca2+-PA complexes submerged into the lipid matrix should provide preconditions for membrane fusion. In such a model of MPT pore, proteins that belong to the megacomplex are to make the membranes to close to each other. The idea of MPT pore to be a gap junction was mentioned at the early stage of MPT exploration (Zoratti and Szabó, 1995), but did not obtain a further development. One of objections was that should such a junction appear, it would never close. However, if the junction is maintained owing to the complexes of Ca2+ with PA dimers, it would be destroyed once Ca2+ cations will have left mitochondria through the pore and the Ca2+-PA complexes will have dissociated.
Thus, the data presented here give a possible explanation of
the mechanism by which PA can induce apoptotic cell death (De Pablo et al.,
1999; Kong and Rabkin, 2000). The studies on the role of SFA in mitochondrial
functions are in progress.
This work
has been support by RFBR (grant).
References:
1. Ardail, D., Privat, J. P., Egret-Charlier, M., Levrat, C., Lerme, F., and Louisot, P. (1990) J. Biol. Chem. 265, 18797-18802
2. Bernardi, P. (1999) Physiol. Rev. 79, 1127-1155
3. Borovyagin, V. L. and Sabelnikov, A. G. (1989) Electron Microsc. Rev. 2, 75-115
4. Broekemeier, K. M., and Pfeiffer, D. R. (1995) Biochemistry 34, 16440-1644926
5. Caman, R. E., Caman, M. W., Hunt, J. M., and Smith, M. S. (1965) J. Neurochem. 12, 15-23
6. De Pablo, M. A., Susin, S. A., Jacotot, E., Larochette, N., Costantini, P., Ravagnan, L., Zamzami, N., and Kroemer, G. (1999) Apoptosis 4, 81-87
7. Gateau-Roesch, O., Pavlov, E., Lazareva, A.V., Limarenko, E.A., Levrat, C., Saris, N.-E.L., Louisot, P. and Mironova, G.D. (2000) J. Bioenerg. Biomembr. 32, 105-110.
8. Haworth, R.A., and Hunter, D.R. (1980) J. Membr. Biol. 54, 231-236
9. Horvath, L., Drees, M., Beyer, K., Klingenberg, M., and Marsh, D. (1990) Biochemistry 29, 10664-10669
10.Kamo, N., Muratsugu, M., Hongoh, R., and Kobatake, Y. (1979) J. Membr. Biol. 49, 105-121
11.Kong J.Y. and Rabkin S.W. (2000) Biochim. Biophys. Acta 1485, 45-55.
12.Levrat, C., and Louisot, P. (1992) Biochem. Biophys. Res. Commun. 183, 719-724
13.Mironova, G.D., Lazareva, A., Gateau-Roesch, O., Tyynela, J., Pavlov, Y., Vanier, M.T. and Saris, N.-E.L. (1997) J. Bioenerg. Biomembr. 29, 561-569.
14.Motulsky, H. (1995) GraphPad guide to Analyzing Radioligand Binding Data p. 4; www.graphpad.com/www/radiolig/radiolig1.htm
15.Mueller, P., Rudin, D.O., Tien H., and Wescott W. (1962) Nature 194, 979-981
16.Petrolini, V., Cola, C., Massari, S., Colonna, R. and Bernardi, P. (1993) J. Biol. Chem. 268, 21939-21945
17.Pfeiffer, D. R., Schmid, P. C., Beatrice, M. C., and Schmid, H. H. O. (1979) J. Biol. Chem. 254, 11485-11494
18.Saris N.-E. L. (1963) Soc. Sci. Fenn.; Comment. Phys.-Math. 28, 1-77
19.Schonfeld P., and Bohnensack R. (1997) FEBS Lett. 420, 167-170
20.Skulachev V. P. (1991) FEBS Lett. 294, 158-162
21.Waters J., Messineo F. C., and Herbette I. G. (1984) J. Biol. Chem. 259, 1319-1324
22.Wharton, D. C., and Tzagoloff, A. (1967) Methods Enzymol. 10, 245-250
23.Wieckowski, M. R., and Wojtczak, L. (1998) FEBS Lett. 423, 339-342
24.Williams, R. J. P. (1976) Symp. Soc. Exp. Biol. 30, 1-17
25.Zoratti, M., and Szabó I. (1995) Biochim. Biophys. Acta 1241, 139-176.