ÔÈÇÈ×ÅÑÊÈÅ ÏÎÄÕÎÄÛ Â ÈÑÑËÅÄÎÂÀÍÈßÕ ÄÍÊ
ßêóøåâè÷ Ë.Â.
(Ïóùèíî)
 äàííîé ðàáîòå îáñóæäàåòñÿ âçàèìîäåéñòâèå ôèçèêè è íàóêè î ÄÍÊ. Ìû ïîêàçûâàåì, ÷òî òàêîå âçàèìîäåéñòâèå ïðèâîäèò ê ïîÿâëåíèþ íîâûõ èíòåðåñíûõ ôèçè÷åñêèõ ìîäåëåé ñ îðèãèíàëüíûìè ãàìèëüòîíèàíàìè, äèíàìè÷åñêèìè óðàâíåíèÿìè è ðåøåíèÿìè.
PHYSICAL MODELS IN DNA SCIENCE
Yakushevich L.V.
(Pushchino)
It is
widely accepted now that physical ideas and methods deeply penetrate to
biology. This process is useful for both physics and biology. But in recent
report made at the Workshop "Biological Physics 2000", Hans
Frauenfelder has noted that although physics and biology have interacted at
least since Galvani, the connection between them looks like a two-way street
with the heavy traffic has gone one way (many tools from physics have been
adopted by researchers in the biological sciences), and the return traffic,
where biological ideas motivate physical considerations, has been less visible
[1].
In this
paper we just consider the "return traffic". As an example, we took
the problem of interaction of nonlinear physics and mathematics with DNA
science, and we discuss what new ideas, physical models, mathematical equations
gave this interaction.
1. New dynamical models
The history
of the penetration of the ideas of nonlinear physics and mathematics to DNA
science began in 1980 when publication of the work of Englander and co-authors
[2] gave a powerful impulse for physicists dealing with nonlinear systems to
study DNA. This led to appearance of many interesting dynamical models. In this
section we describe some of them.
From the
first glance DNA is nothing but a system consisting of many atoms interacting
with one another and organized in a special way in space. Under usual external
conditions (temperature, pH, humidity, etc) this space organization has the
form of the double helix which is rather stable but moveable system. The
thermal bath where the DNA molecule is usually immersed is one of the reasons
of the DNA internal mobility. Collisions with the molecules of the solution
which surrounds DNA, local interactions with proteins, drugs or with some other
ligands also lead to internal mobility. As a result, different structural
elements of the DNA molecule such as individual atoms, groups of atoms (bases,
sugar rings, phosphates), fragments of the double chain including several base
pairs, are in constant movement. So, from the point of physics, the DNA
molecule can be considered as a complex dynamical system with large number of
internal motions.
How to
model the system? Physicists proposed many dynamical models. The main models
were gathered in [3, 4] and we present them here in the table 1. For
convenience the models are arranged in the order of increasing their complexity
and each new level of complexity is presented as a new line in the table.
Table 1. Approximate models of DNA structure
and dynamics.
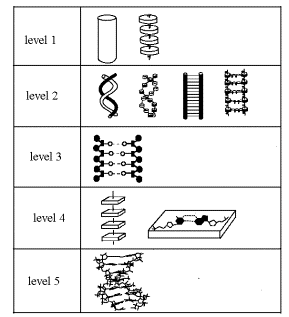
In the
first line of the table, the simplest models of DNA, namely, the model of
elastic thread and its discreet version, are shown. These models are too simple
and they are not original.
In the
second line of the table, more complex models of the internal DNA dynamics are
shown. They take into account that the DNA molecule consists of two
polynucleotide chains. The first of the models consists of two elastic threads
weakly interacting with one another and being wound around each other to
produce the double helix. The discreet version of the model is nearby. The next
two models in the line are simplified versions of the previous two models, which
are often used by investigators. In these models the helicity of the DNA
structure is neglected. The model of this line looks rather original. They have
not any analogy in physics.
In the
third line a more complex model of the DNA internal dynamics is shown. It takes
into account that each of the chains consists of three types of atomic groups
(bases, sugar rings, phosphates). In the Table 1 different groups are shown
schematically by different geometrical forms, and, for simplicity, the helicity
of the structure is omitted.
The models
of this line have not any analogy in physics too.
The list of
models could be continued and new lines with more and more complex models of
DNA structure and dynamics could be added till the most accurate model which
takes into account all atoms, motions and interactions, would be reached. And
there are not doubts that all of them are very original and have not analogy in
physics.
2. New mathematical models
Let us
illustrate now that interaction of DNA science with nonlinear mathematics does
lead to new mathematical equations with new interesting solutions. For the
purpose, we can refer to our previous work [5] where a new model hamiltonian
and nonlinear dynamical equations describing internal DNA dynamics (in the
framework of the models of second line of the table 1) have been obtained. Improved
version of the hamiltonian, which has been recently obtained, is
H = (m/2) Sn {[(dun,1/dt)2 + (R0
– un,1)2 (dQn,1/dt)2 + m(dzn,1 /dt)2]
+ [(dun,2/dt)2
+ (R0 + un,2)2 (dQn,2/dt)2+ m(dzn,2 /dt)2]}
+
+ (K/2) Sn {[ 2R20 [1- cos(Qn,1 -Qn-1,1 )] + u2n,1+ u2n-1,1
– 2 un,1 un-1,1 cos(Qn,1 – Qn-1,1 ) –
-2 R0 un,1
[1 – cos(Qn,1 -Qn-1,1 )] – 2 R0 un-1,1 [1 –
cos(Qn,1 -Qn-1,1 )] + |zn,1 – zn-1,1 |2
+
+ |zn,2
– zn-1,2 |2] + [2R20 [1- cos(Qn,2 -Qn-1,2 )] + u2n,2+ u2n-1,2
–
- 2 un,2
un-1,2 cos(Qn,2 – Qn-1,2 ) + 2 R0 un,2 [1 – cos(Qn,2 -Qn-1,2 )] +
+ 2 R0
un-1,2 [1 – cos(Qn,2
-Qn-1,2 )]]} + (k /2 )Sn {[ 2R02{(1 – 2 cosQn,1) +
+ (1 – 2cosQn,2 ) + [1 + cos(Qn,1 -Qn,2)] } – 2R0 un,1 (1 – 2
cosQn,1)+
+ 2R0 un,2
(1 – 2 cosQn,2) + un,1 2 + u2n,2
– 2 un,1 un,2 cos(Qn,1 – Qn,2 ) –
- 2R0 un,1 cos(Qn,1 – Qn,2 ) + 2R0 un,2 cos(Qn,1 -Qn,2)] + k |zn,1 – zn,2 |2 } ; (4)
where Qn,i describes angular displacement of the n-th
structural unit of the i-th chain; un,i describes the transverse
displacement; zn,i describes the longitudinal displacement (i =
1,2); m is a common mass of nucleotides; K is the coupling constant along each
strand; R0 is the radius of DNA; a is the distance between bases
along the chains; and V is the potential function describing interaction
between bases in pairs.
Hamiltonian
(4) can be written in a more convenient form
H = H(f) + H(Y) + H(g) + H(interact.); (5)
where
H(f) = (m R20/2)
Sn (dfn,1/dt)2 + (m R20/2)
Sn (dfn,2/dt)2 + (K R20/2)
Sn (fn,1- fn-1,1)2
+
+ (K R20/2) Sn (fn,2 – fn-1,2)2 + (k R20 /2 ) Sn (fn,1 + fn,2)2; (6)
H(Y) = (m R20/2) Sn (dYn,1/dt)2 + (m R20/2) Sn (dYn,2/dt)2 +
+ (KR20)
Sn [1- cos(Yn,1 – Yn-1,1 )] + + (KR20) Sn [1- cos(Yn,2 -Yn-1,2 )] +
+ (kR02) Sn { 2 (1- cosYn,1) + 2 (1- cosYn,2 ) – [1 – cos(Yn,1 +Yn,2)]}; (7)
H(g) = (m R20/2) Sn (dgn,1/dt)2 + (m R20/2)
Sn (dgn,2/dt)2 + (K R20/2)
Sn (gn,1- gn-1,1)2
+
+ (K R20/2) Sn (gn,2 – gn-1,2)2 + (k R20 /2 ) Sn (gn,1 + gn,2)2; (8)
H(interact.) = (m R20/2)
Sn (- 2 fn,1 + f2n,1)
(dYn,1/dt)2 +
+ (m R20/2) Sn (-2fn,2 + f n,22)(dYn,2/dt)2 +
+ (K R20) Sn [1-cos(Yn,1 – Yn-1,1 )] [fn,1 fn-1,1 – fn-1,1
– fn,1 ] +
+ (K R20) Sn [1-cos(Yn,2 – Yn-1,2 )] [fn,2 fn-1,2 – fn,2
– fn-1,2] -
- (2k R20) Sn (fn,1) (1 – cosYn,1) – (2k R20) Sn (fn,2 ) (1 – cosYn,2)+
+ (k R20) Sn (-fn,1 fn,2 + fn,1 + fn,2 ) [1-cos(Yn,1 + Yn,2)]; (9)
with new variables
fn,1=
un,1/R0; fn,2
= -un,2/R0;
Yn,1=Qn,1; Yn,2= -Qn,2; (10)
gn,1=
zn,1/R0; gn,2
= -zn,2/R0.
Here H(f) describes transverse motions; H(Y) describes torsional motions; H(g) describes
longitudinal motions; H(interact.) describes interactions between the motions.
Usually it
is suggested that the solutions are rather smooth functions (that is the
functions f1, f2, g1, g2, Y1, Y1 change substantially only at the distances
which are much more than the distance between neighboring base pairs), and
continuos approximation is used. In the continuos approximation the model
hamiltonian takes the form
Hcont. = (rm R20/2) ò dz [(¶f1/¶t)2 + (¶f2/¶t)2] + (Y R20/2)
ò dz [(¶f1/¶z)2 + (¶f2/¶z)2] +
+ (y R20 /2 ) ò dz (f1 + f2)2
+ (rm R20/2) ò dz [(¶g1/¶t)2 + (¶g2/¶t)2] +
+ (Y R20/2) ò dz [(¶g1/¶z)2 + (¶g2/¶z)2] + (y R20
/2 ) ò dz (g1 + g2)2
+
+ (rm R20/2) ò dz [(1- f1 )2
(¶Y1/¶t)2 + (1- f2 )2(¶Y2/¶t)2] +
+ (Y R20/2) ò dz [(1- f1 )2
(¶Y1/¶z)2 + (1- f2 )2 (¶Y2/¶z)2] +
+ (y R02) ò dz {2 (1-f1) (1- cosY1) + 2 (1-f2) (1- cosY2 ) +
+ (-f1 f2 + f1 + f2-1 ) [1 – cos(Y1 +Y2)]}; (11)
where m/a = rm; Ka = Y; k/a = y. And the dynamical
equations which correspond to the model hamiltonian (11), can be easily
obtained from the general theory of hamiltonian systems
rm (d2 f1/dt2) + rm (1- f1) (dY1/dt)2 = Y ¶2f1/¶z2 + Y (¶Y1/¶z)2(1 – f1) –
– y (f1 + f2) + 2y (1 – cosY1) – y (1 – f1)[1-cos(Y1 + Y2)] ; (12)
rm (d2 f2/dt2) + rm (1- f2) (dY2/dt)2 = Y ¶2f2/¶z2 + Y (¶Y2/¶z)2(1 – f2) –
– y (f1 + f2) + 2y (1 – cosY2) – y (1 – f2 )[1-cos(Y1 + Y2)] ; (13)
rm (1 – f1) (d2Y1/dt2) – 2rm (df1/dt) (dY1/dt) = Y (¶2Y1/¶z2) (1- f1) –
– 2Y (¶Y1/¶z) [¶f1/¶z ] – 2y [(sinY1)] + y(1 – f1)[sin(Y1 + Y2)] ; (14)
rm (1 – f2) (d2Y2/dt2) – 2rm (df2/dt) (dY2/dt) = Y (¶2Y2/¶z2) (1- f2) –
– 2Y (¶Y2/¶z) [¶f2/¶z] – 2y [(sinY2 )] + y(1 – f1)[sin(Y1 + Y2)]. (15)
rm (d2 g1/dt2) = Y ¶2g1/¶z2 – y (g1 + g2); (16)
rm (d2 g2/dt2) = Y ¶2g2/¶z2 – y (g1 + g2). (17)
3. New models of inhomogeneity
Many
different models of inhomogeneity are used in physics. The most popular of them
are point inhomogeneity, boundary between two neighboring homogeneous ranges
and random inhomogeneity. DNA gives us a new type of inhomogeneity, which is
appeared due to the sequence of bases. Four types of bases (adenine, thymine,
guanine and cytosine) forms the sequence which is specific and unique for any
living organism.
In the
models considered above, we ignored the difference between the bases. That is
we considered the models like this
b b b b b b b b b b b
| | | | | | | | | | | (1)
b b b b b b b b b b b
where b denotes a base.
But model
(1) is not correct even for homogeneous fragment of DNA (for example for
synthetic polyA·polyT).
Correct variant is
AAAAAAAAAAA
| | | | | | | | | | | (2)
TTTTTTTTTTTT
We could
say that this fragment of DNA looks like a quasi-one dimensional crystal with
two "atoms" (nucleotides) in the cell.
In real
DNA, however, we have a sequence like this
AGCTTCGAAGG
| | | | | | | | | | | (3)
TCGAAGCTTCC
This type
of inhomogeneity is very unusual from physical point of view.
6. Conclusions
In this
paper we tried to show that interaction between nonlinear physics and DNA
science looks rather promising not only for biology, but also for physics. We
presented several examples to illustrate this statement. The list of examples
could be continued by including many other interesting problems that appear due
to the interaction. The problem of DNA-surrounding interaction, the problem of
statistics of local distortions (unwound regions, for example) in DNA, the
problem scattering of light and neutrons by DNA are only some of them. Moreover,
we are sure that further investigations will bring us new examples confirming
the statement.
References:
1.
H.
Frauenfelder Abstracts of The First
Workshop on Biological Physics 2000 (September 18-22, 2000, Bangkok, Thailand)
p. 13, 2000.
2.
S.W.
Englander, N,R. Kallenbach, A.J. Heeger, J.A. Krumhansl and A. Litwin, Proc. Natl. Acad. Sci. USA 77, 7222, 1980.
3.
L.V.
Yakushevich Quart. Rev. Biophys. 26, 201, 1993.
4.
L.V.
Yakushevich Nonlinear physics of DNA.
Wiley, N.Y., 1998.
5. L.V. Yakushevich Mathematics. Computer. Education. 7, 696, 2000.