НЕЛИНЕЙНЫЕ ВОЛНЫ В ДНК. АНАЛИТИЧЕСКИЕ ИССЛЕДОВАНИЯ И
КОМПЬЮТЕРНОЕ МОДЕЛИРОВАНИЕ
Якушевич
Л.В., Савин А.В., Маневич Л.И.
(Пущино, Москва)
Обсуждаются и сравниваются результаты аналитических
исследований и компьютерного моделирования нелинейных конформационных волн в
ДНК. Такие волны являются решениями уравнений, описывающих вращательные
движения оснований большой амплитуды. Показано, что с помощью компьютерных
методов удается получить новые нелинейные волновые решения, которые не
удавалось получить аналитическими методами. При помощи компьютерных методов
удалось также показать, что нелинейные волновые решения обладают устойчивостью
по отношению к тепловым колебаниям, и уточнить поведение этих волн при
пересечении границы двух однородных областей (АТ и GC).
NONLINEAR WAVES IN
DNA. ANALYTICAL STUDIES AND COMPUTER SIMULATIONS
Yakushevich L.V.,
Savin A.V., Manevitch L.I.
(Pushchino, Moscow)
We discuss and compare the results of analytical and
computer studies of the nonlinear conformational waves in DNA. These waves are
the solutions of the equations describing large-amplitude rotational motions of
DNA bases. It is shown that computer methods permit to obtain new nonlinear
wave solutions, which can not be found by analytical methods. Computer methods
permit also to show the stability of the solutions in the thermalized double
chain and to define more precisely the dynamical behavior of the nonlinear
waves when they are crossing the boundary between two homogeneous regions (AT
and GC).
Introduction
In accordance with recent investigations, the DNA molecule is considered here as a moveable system with many types of internal motions [1,2]. Among others large-amplitude rotational motions of bases are of most interest because they can lead to unwinding the DNA double helix. Analytical studies of the differential equations imitating the motions, show that the equations have nonlinear wave solutions which can be interpreted as unwound regions moving along the DNA [3]. New interesting results have been obtained recently by numerical methods [4]. Here we discuss and compare the results of analytical and computer studies. We show that numerical methods permit to obtain new nonlinear wave solutions for asymmetrical model of DNA, which can not be obtained by analytical methods. Methods of computer modeling permit to show the stability of the solutions in the thermalized double chain and to define more precisely the dynamical behavior of the nonlinear waves when they are crossing the boundary between two homogeneous regions (AT and GC).
Model hamiltonian
The first model hamiltonian imitating large-amplitude rotational motions of bases was proposed by Englander and co-authors [5]. This work gave an impulse for a large group of authors to improve the model (see references in the review [6] and in the book [2]). Here we present the model hamiltonian derived recently in our paper [3]. In contrast to other works, we suggest that bases in pairs are not identical and the model is therefore asymmetrical.
For simplicity, we let us write here the model hamiltonian for
homogeneous double chain consisting of only A-T base (fig.1).
We suggest that the hamiltonian consists of three parts
H = T + V|| + V^; (1)
where T describes the kinetic energy
T = Sn {(mAr2A/2) (djn,A/dt)2 + (mTr2T/2) (djn,T/dt)2}; (2)
V|| describes the energy of interactions along the DNA
V|| = Sn {(KAr2A) [1- cos(jn,A - jn-1,A)] + (KTr2T) [1- cos(jn,T - jn-1,T)]}; (3)
And V^ describes the energy of interactions of bases in pairs
V^ = Sn (kA-T) {rA(rA +
rT)(1 - cosjn,A) + rT (rA + rT)(1
- cosjn,T) -
- rArT [1 - cos(jn,A - jn,T)]}. (4)
Here jn,i is the angular displacement of the n-th base of the i-th chain from its equilibrium position; ri is the distance between bases of the i-th chain and the nearest sugar-phosphate backbone; a is the distance between neighboring bases along the chains; mi is mass of bases of the i-th chain; Ki is the coupling constant along the sugar-phosphate chain; n = 1, 2, … N; i = A, T.
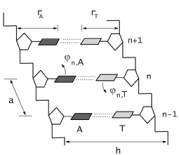
Fig. 1. Homogeneous double chain
consisting of AT base pairs.
Dynamical equations and their
analytical solutions
According to the general principles of theoretical mechanics, the dynamical equations corresponding to hamiltonian (1) can be found from the equations of Hamiltonian
d/dt {Pn,i}= -¶H/¶jn,i; (5)
with impulse Pn,i determined by formula
d/dt {Pn,i} = ¶L/¶jn,i; (6)
and the function of Lagrange determined by
L = T - V|| - V^. (7)
Inserting hamiltonian (1) into (5), we obtain the dynamical equations
mA rA2(d2jn,A/dt2) =
= KArA2[sin(jn-1,A - jn,A
) - sin(jn,A
- jn+1,A)]
-
- kA-T[2rA(rA+rT)sinjn,A - rTrAsin(jn,A-jn,T)] = 0; (8)
mT rT2(d2jn,T/dt2) =
= KTrT2[sin(jn-1,T - jn,T)
- sin(jn,T
- jn+1,T)]
-
- kA-T[2rT(rA+rT)sinjn,T - rTrAsin(jn,T-jn,A)] = 0.
Solutions of the equations can be found analytically in symmetrical case when
mn,i @ m; Ki @ K; Rn,i @ R; kn @ k. (9)
Indeed, in this case the dynamical equations are reduced to
m(d2jn,A/dt2) + K[sin(jn,A - jn-1,A ) - sin(jn+1,A - jn,A)] +
+ k[2 sinjn,A - sin(jn,A - jn,T)] = 0; (10)
m(d2jn,T/dt2) + K[sin(jn,T - jn-1,T) - sin(jn+1,T - jn,T)] +
+ k[2 sinjn,T - sin(jn,T - jn,A)] = 0. (11)
Suggesting that the solutions of the equations are rather smooth functions, we can rewrite them in the continuos approximation
m(¶2jA/¶t2) - Ka2(¶2jA/¶z2) + k[(2(sinjA) - sin(jA - jT)] = 0; (12)
m(¶2jT/¶t2) - Ka2(¶2jT/¶z2) + k[2(sinjT) - sin(jT - jA)] = 0. (13)

Fig. 2. Local unwound region moving along the double chain. Long lines are used to show schematically sugar-phosphate chains, and short lines - to show bases.
Equations (12) - (13) have the form of modified sine-Gordon equations. So, among others they have nonlinear wave solutions (or soliton-like solutions) [7]
jA(z-vt) = jT(z-vt) = 4 arctan{exp[g(x-x0)/d]}; (14)
where g = [1 - (m/Ka2) v2]-1/2; x = z-vt; v is a constant velocity of the soliton and d = a (K/2k)1/2 is its size. The solution can be interpreted as that describing the formation of unwound region in the DNA double helix. In Fig. 2 qualitative picture which corresponds the solution, is presented. If we take KR2= (0,2¸2)´10-11 erg [8], R = 10 Å; a = 3,4 Å and k = 26´103 dyn/cm = 26´103 g/sec2 [9], then the value of the size of the local distortion can be estimated as d = {21,08¸66,67}Å. This result well correlates with experimental data, namely, with the size of local distortion due to DNA protein binding (for example, binding of RNA-polymerase with promoter regions).
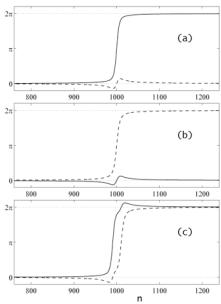
Fig. 3. Soliton solutions with the topological charges q = (1,0) (a), q = (0,1) (b), q = (1,1) (c). Continuous lines correspond to displacements by the first component jn,A, dotted lines - to displacements by the second component jn,T.
In asymmetrical case when
mn,i ¹ m; Ki ¹ K; Rn,i ¹ R. (15)
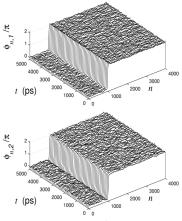
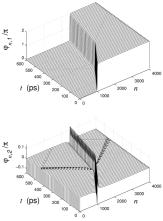
the solutions of equations (8) can be found numerically by using variation technique, proposed in [10]. The results obtained are shown in fig. 3. They demonstrate that in asymmetrical case more types of soliton-like solutions having different topological charge are possible. Investigations of the interaction of the solutions with to thermal oscillations show that that they are rather stable with respect to thermal oscillations [4]. One of the examples confirming this statement is presented in fig. 4. Interactions of the solutions with one another and with different types of inhomogeneities such as point inhomogeneities, random sequence of bases, boundary between two homogeneities regions, are of most interest. One of the results is shown in fig. 5 where the movement of soliton-like solution through the boundary between two homogeneous regions (AT and GC) is presented. The result differs from that obtained earlier by analytical methods which permit to consider only symmetrical case when the difference between bases in pairs is neglected [11]. So, according to numerical results, the value of the velocity of the movement of nonlinear waves in the AT region is larger than in the GC region.
Discussion
In this paper we described shortly the main results of analytical and numerical studies of the nonlinear conformational waves in DNA. We showed that numerical methods permit to obtain more types of nonlinear waves in DNA; to study the stability of the waves, to define more precisely the dynamical behavior of the nonlinear waves when they are crossing the boundary between two homogeneous regions (AT and GC).
|
Fig. 4. Stability of topological soliton (q=(1,1)) in the
thermalized homogeneous AT chain (T = 300K) |
Fig. 5. Movement of soliton (q= (1,0)) through the boundary between two homogeneous AT and
GC regions. |
Bibliography
1.J.A. McCommon and S.C. Harvey, Dynamics of proteins and nucleic acids, Cambridge University Press, Cambridge, 1987.
2.L.V. Yakushevich, Nonlinear physics of DNA, Wiley & Sons, Chichester, 1998.
3.L.V. Yakushevich, J. Biosci. 26, 305, 2001.
4.Л.В. Якушевич, А.С. Савин и Л.И. Маневич, в сб. Нелинейные явления в открытых системах, ред. Лупичев Л.Н., вып. 12, Москва, 2001, стр. 3-42.
5.S.W. Englander, N.R. Kallenbach, A.J. Heeger, J.A. Krumhansl and A. Litwin, Proc. Natl. Acad. Sci. USA 77, 7222, 1980.
6.L.V. Yakushevich, Quart. Rev. Biophys. 26, 201, 1993.
7.L.V. Yakushevich, Russian J. Phys. Chemistry 69, 1277, 1995.
8.V.K. Fedyanin and L.V. Yakushevich, Stud. biophys. 103, 171, 1984.
9.K. Itoh and T. Shimanouchi, Biopolymers 9, 383, 1970.
10.P.L. Christiansen, A.V. Zolotaryuk and A.V. Savin, Phys. Rev. E 56, 877, 1997.
11.L.V. Yakushevich Studia biophys. 121, 201, 1987.